As the electronics industry continues its shift toward lead-free manufacturing – a transition catalyzed by the 2006 RoHS Directive, electrically conductive adhesives (ECAs) – especially silver-filled epoxies, have emerged as a reliable alternative to traditional soldering techniques. However, their performance and long-term reliability are not inherent; they are critically dependent on compatible metallization.
Choosing the wrong substrate metal seeds predictable failure mechanisms that can evade initial testing. This post breaks down the science behind silver epoxy bonding, details the significant risks of incompatible metals, and provides clear guidelines for making smart, reliable metallization choices.
Why Use Silver Epoxies?
Silver epoxies have been used in semiconductor and electronic packaging since the 1960s. They’re valued for:
- High electrical conductivity
- Compatibility with temperature-sensitive components
- Flexibility across diverse substrates and devices
Since the early 2000s, the global push toward lead-free electronics has made silver ECAs even more essential—particularly as manufacturers move toward pure tin or tin-rich alloys for soldering and surface finishes.
Understanding the core failure mechanisms: More than just a “bad bond”
To make intelligent material choices, it’s crucial to understand the science behind the failures.
- Galvanic corrosion: This is the primary failure mode for incompatible pairs. When silver (a noble metal) is bonded to a more active metal like tin or aluminum in the presence of even trace moisture, they form an electrochemical cell. The active metal corrodes, which leads to increased electrical resistance, the generation of non-conductive corrosion products, and eventual bond failure.
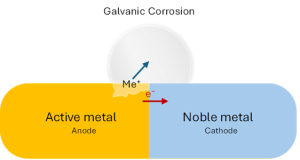
Schematic diagram of galvanic corrosion mechanism
- Tin Whisker Growth: A major risk with pure tin surfaces is the spontaneous growth of conductive “whiskers”—needle-like metal crystals. Factors like stress (from the adhesive or thermal cycling) and the presence of silver as a catalyst can accelerate this growth. These whiskers can bridge conductors, causing catastrophic short circuits, and have been linked to high-profile failures in satellites, medical devices, and missile systems.
- Non-Conductive Oxide Formation: Metals like aluminum rapidly form a surface oxide (Al₂O₃), which is an electrical insulator. Bonding to this layer results in high initial resistance and can reduce adhesive lap shear strength by up to 50%.
Compatibility: What Metals work and why?
Successful bonding requires matching silver with other noble or properly prepared metals. Here’s a practical breakdown:

Table of galvanic series:
| Compatibility | Metals | Why we need this and what it’s for |
| Recommended | Gold (Au), Platinum (Pt), Palladium (Pd), Silver (Ag), AgPd | Noble metals that resist oxidation, ensuring stable, conductive interfaces.
This is an ideal alternative to solder joining, yielding similar thermal conductivity. |
| Use with Care | Nickel (Ni), Copper (Cu) | Nickel requires passivation or a noble metal top-coat (as in ENIG finishes) to prevent surface oxidation and ensure a stable interface.
Copper must be thoroughly cleaned to remove oxides immediately before bonding. |
| Avoid | Tin (Sn), Aluminum (Al), Solder Alloys (SnPb, SnAgCu) | Tin creates galvanic corrosion and whisker risk.
Aluminum oxide severely weakens adhesive bonds—reducing lap shear strength by up to 50%. |
Do Not Use Silver Epoxy With: Pure tin finishes, SnAgCu solder pads, solder-dipped
Environmental Threat: Gas-Phase corrosion
Selecting compatible metals ensures a sound initial bond, but long-term reliability requires defending that bond against its operating environment. Beyond interfacial corrosion, the silver filler within the epoxy itself can be attacked by atmospheric pollutants, a process known as gas-phase corrosion.
- The Culprits: Reactive gases like hydrogen sulfide (H₂S), sulfur dioxide (SO₂), chlorine (Cl₂), and nitrogen oxides (NOₓ) are common in industrial, urban, agricultural, and coastal environments.
- The Mechanism: In the presence of humidity, these gases dissolve into the thin moisture layer on a surface, forming localized acidic electrolytes (e.g., sulfuric or hydrochloric acid). This process aggressively attacks susceptible metals.
- Silver’s Specific Vulnerability: Silver is highly reactive with sulfur compounds. As cited in corrosion research at below, even low levels of hydrogen sulfide (H₂S) can cause severe corrosion of silver conduction lines. This attack leads to thinning of the lines, an increase in resistance, and the formation of corrosion products, including dendrites, which can compromise circuit integrity.
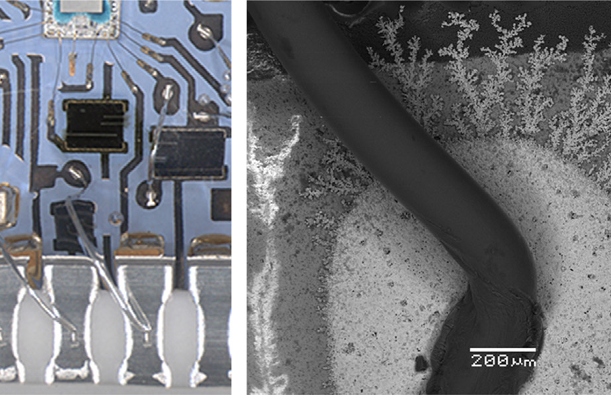
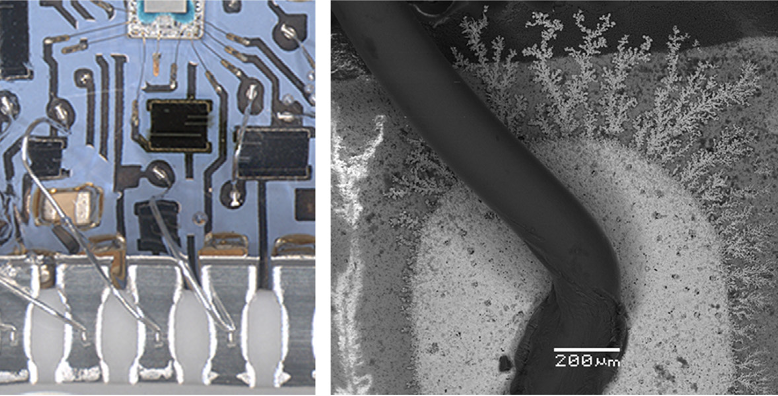
A Telling Case Study: Research documented the failure of a silicone-gel-coated hybrid circuit in a pig farm atmosphere rich in H₂S. Despite the thick protective gel, humidity and gas permeated the coating, corroding silver conduction paths, increasing line resistance, and forming dendrites. This underscores a key principle: passive protection alone is often insufficient against persistent environmental threats.

Corrosion of silver conduction lines on a hybrid PCBA exposed to pig farm conditions with H2S: (A) overview of corroded silver lines (black layer on silver is due to corrosion) and (B) formation silver sulfide dendrite due to the movement of corrosion products at the edge of the conduction paths.
Compatibility Chart: Where Silver Epoxies Work Best
Selecting the right finish depends on your industry’s demands. The chart below summarizes key recommendations:
| Market/ Sector | Recommended Metallization | Primary Applications |
| Semiconductor & Hybrid Micro-Electronics | Pd, Ni/Pd/Au, Ag, Alloy 42, AgPd, Brass, SST, Kovar | Wafers-level packaging, lead frames, die attach. Avoid Sn-plated lead frame & Cu die paddles. |
| General Electronics Assembly | Au, Cu (cleaned), PTF-Ag ink, PZT, SnO, ZnO, Al/Cu, Cu/Sn, Cu/Ag, Mo, Ni, Cr, TCOs | Pads on PCB, SMD capacitor attach, antennae, RFIDs. |
| Medical Devices & High Reliability | Au/Ceramic, Pt/Ir | Pacemakers, catheters, implantable sensors. |
| Opto-electronics & Automotive | Brass, SST, Kovar, Au/ceramic, lithium niobate, Cu, ITO | LED die attach, optical sensors, under-hood modules. Optical components attach, EMI shielding, metal housings, Si, GaAs, InP, and MEMS. |
Building a reliable process: Beyond Material Selection
Choosing a compatible metal is only the first step. Ensuring long-term reliability requires controlled processes:
- Surface cleanliness: Ionic flux residues are the most common electrolyte for galvanic corrosion. Implement validated cleaning processes, even with “no-clean” fluxes.
- Process control: Bond in a controlled environment (<60% RH) to minimize moisture uptake. Use gloves to prevent chloride contamination from fingerprints.
- Conformal coatings & enclosures: Sealing assemblies to limit atmospheric exposure. We are pleased to announce the launch of our epoxy-based series of conformal coatings, which are proven to be highly effective in protecting against chemical and environmental attacks.
- Environmental control: Using filtered air or controlled atmospheres in storage and assembly.
Conclusion
Choosing the right metallization is just as important as selecting the right adhesive. For silver epoxy ECAs to perform reliably, they must be paired with noble metals that resist oxidation and promote strong, conductive bonds.
Questions about compatibility? Our experts can help you evaluate your materials and recommend the right ECA for your application.
Email techserv@epotek.com